New Design Rule for High-Entropy Superionic Solid-State Conductors
Solid electrolytes with high lithium-ion conductivity can be designed for millimeter-thick battery electrodes by increasing the complexity of their composite superionic crystals, report researchers from Tokyo Tech. This new design rule enables the synthesis of high-entropy active materials while preserving their superionic conduction.
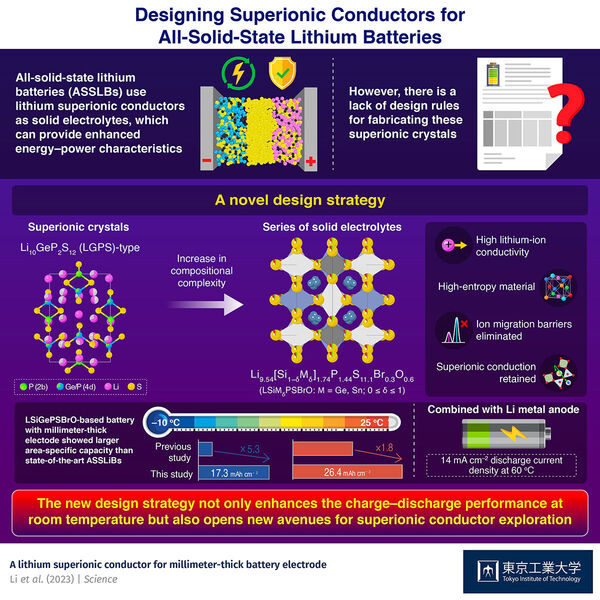
As the world transitions towards a greener and more sustainable energy economy, reliance on lithium (Li)-ion batteries is expected to rise. Scientists from across the globe are working towards designing smaller yet efficient batteries that can keep up with the ever-increasing demand for energy storage. In recent years, all-solid-state lithium batteries (ASSLBs) have captured research interest due to their unique use of solid electrolytes instead of conventional liquid ones. Solid electrolytes not only make the battery safer from leakage and fire-related hazards, but also provide superior energy and power characteristics. However, their stiffness results in poor wetting of the cathode surface and a lack of homogenous supply of Li ions to the cathode. This, in turn, leads to a loss of capacity in the solid-state battery. The issue becomes more pronounced in thick battery cathode electrode such as millimeter-thick one, which is a more advantageous electrode configuration for realizing inexpensive and high-energy-density battery package, compared to conventional electrode with typical thickness of < 0.1 mm.
Fortunately, a recent study published in Science found a way to overcome this problem. The paper--authored by a team of researchers led by Prof. Ryoji Kanno from Tokyo Institute of Technology (Tokyo Tech)--describes a new strategy to produce solid electrolytes with enhanced Li-ion conductivity. Their work establishes a design rule for synthesizing high-entropy crystals of lithium superionic conductors via the multi-substitution approach.
"Many studies have shown that inorganic ionic conductors tend to show better ion conductivity after multi-element substitution probably because of the flattened potential barrier of Li-ion migration, which is essential for better ion conductivity," points out Prof. Kanno. This was where they started their research. For the design of their new material, the team took inspiration from the chemical compositions of two well-known Li-based solid electrolytes: argyrodite-type (Li6PS5Cl) and LGPS-type (Li10GeP2S12) superionic crystals. They modified the LGPS-type Li9.54Si1.74P1.44S11.7Cl0.3 via multi-substitution and synthesized a series of crystals with composition Li9.54[Si1−δMδ]1.74P1.44S11.1Br0.3O0.6 (M = Ge, Sn; 0 ≤ δ ≤ 1).
The researchers used a crystal with Ge = M and δ = 0.4 as a catholyte in an ASSLB with an 1- or 0.8- millimeter-thick cathode. The former and latter ASSLB exhibited discharge capacities of 26.4 mAh cm−2 at 25℃ (1 mm) and 17.3 mAh cm−2 at −10 ℃ (0.8 mm), respectively, with the area-specific capacity 1.8 and 5.3 times larger than those reported for previous state-of the-art ASSLBs, respectively. Theoretical calculations suggested that the enhanced conductivity of the solid electrolyte could be a result of the flattening of the energy barrier for ion migration, caused by a small degree of chemical substitution in the above-mentioned crystal.
This study provides a new way for preparing high-entropy solid electrolytes for millimeter-thick electrodes while preserving their superionic conduction pathways. "In effect, the proposed design rule lays a solid groundwork for exploring new superionic conductors with superior charge-discharge performance, even at room temperature," concludes Prof. Kanno.
Reference
Authors : Yuxiang Li1, Subin Song2, Hanseul Kim2, Kuniharu Nomoto1, Hanvin Kim1, Xueying Sun2, Satoshi Hori1, Kota Suzuki1, Naoki Matsui1, Masaaki Hirayama1,2, Teruyasu Mizoguchi3, Takashi Saito4,5,6, Takashi Kamiyama4,6, and Ryoji Kanno1,*
Title : A lithium superionic conductor for millimeter-thick battery electrode
Journal : Science
Affiliations : Yuta Yasui1 Masataka Tansho2, Kotaro Fujii1, Yuichi Sakuda1, Atsushi Goto2, Shinobu Ohki2, Yuuki Mogami2, Takahiro Iijima3, Shintaro Kobayashi4, Shogo Kawaguchi4, Keiichi Osaka5, Kazutaka Ikeda6,7,8, Toshiya Otomo6,7,8,9, Masatomo Yashima1*
* Corresponding author
Affiliations :
1 Research Center for All-Solid-State Battery, Institute of Innovative Research, Tokyo Institute of Technology
2 Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology
3 Institute of Industrial Science, the University of Tokyo
4 Neutron Science Division (KENS), Institute of Materials Structure Science, High Energy Accelerator Research Organization (KEK)
5 Department of Materials Structure Science, School of High Energy Accelerator Science, The Graduate University for Advanced Studies
6 Japan Proton Accelerator Research Complex (J-PARC) Center, Materials and Life Science Division